Consider the gas phase reaction NO + 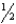 O2
O2  NO2 for which H° = -56.91 kJ and K = 1.46 106 at 25°C.
NO2 for which H° = -56.91 kJ and K = 1.46 106 at 25°C.
-Calculate H° at 25°C for the following reaction: 2NO + O2  2NO2
2NO2
A) 56.91 kJ
B) -113.8 kJ
C) -28.5 kJ
D) 3239 kJ
E) none of these
Correct Answer:
Verified
Q76: Which of the following statements is
Q77: When a stable diatomic molecule spontaneously
Q78: Which of the following is true
Q79: In which process is
Q80: Of
Q82: Consider the gas phase reaction NO
Q83: Determine
Q84: Given that
Q85: Consider the gas phase reaction NO
Q86: The following reaction has a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents