Consider the voltaic cell based on the following reaction: 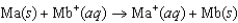
Two students are assigned to measure the standard cell potential. Yvonne claims that both solutions of ions have to be at exactly 1.00 M concentration, but Zelda is sure that the measurement will be the same if the concentrations of the two solutions are equal, but not necessarily 1.00 M. What do you think? (The temperature is controlled at 25 C, so that's not an issue.)
A) Yvonne is right because by definition standard cell potentials must be measured at concentrations of 1.00 M.
B) Yvonne is right because she understands the Nernst equation and what it describes.
C) Zelda is right because she understands the Nernst equation and what it describes.
D) Zelda is right because cell potentials do not depend on the concentration.
E) Both are right because cell potentials do not depend on the concentration.
Correct Answer:
Verified
Q73: If the free-energy change of a voltaic
Q74: Which statement does not correctly describe a
Q75: Does pH have an effect on the
Q76: The standard hydrogen electrode is _
A)used to
Q77: The numerical value of the Faraday constant
Q79: Which statement does not correctly describe a
Q80: If the potential of a voltaic cell
Q81: An electrochemical cell is represented below. Which
Q82: The standard cell potential for the nickel-cadmium
Q83: What is true when a battery (voltaic
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents