Which statement does not correctly describe a standard hydrogen electrode (SHE) ?
A) The SHE is assigned a standard reduction potential of exactly 1 V.
B) 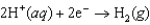
C) Pt|H2(g, 1atm) |H+(aq, 1 M ) ||
D) ||H+(aq, 1 M ) |H2(g, 1atm) |Pt
E) The SHE consists of a platinum electrode immersed in an acid solution and a stream of hydrogen gas.
Correct Answer:
Verified
Q69: If the free-energy change of the following
Q70: The change in free energy for
Q71: Using the following data, determine the standard
Q72: The Nernst equation can be used to
Q73: If the free-energy change of a voltaic
Q75: Does pH have an effect on the
Q76: The standard hydrogen electrode is _
A)used to
Q77: The numerical value of the Faraday constant
Q78: Consider the voltaic cell based on
Q79: Which statement does not correctly describe a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents