If the free-energy change of the following voltaic cell 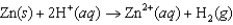 is -147.2 kJ, what is the standard potential of the cell?
is -147.2 kJ, what is the standard potential of the cell?
A) (+0.763 V)
B) (-0.763 V)
C) (+1.53 V)
D) (-1.53 V)
E) (+2.12 V)
Correct Answer:
Verified
Q64: An electrochemical cell with a standard
Q65: The spontaneous redox reaction in a
Q66: The work involved in moving exactly 1
Q67: If the potential of a voltaic cell
Q68: Copper is oxidized by nitric acid. If
Q70: The change in free energy for
Q71: Using the following data, determine the standard
Q72: The Nernst equation can be used to
Q73: If the free-energy change of a voltaic
Q74: Which statement does not correctly describe a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents