Using the following data, determine the standard cell potential 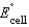 for the electrochemical cell constructed using the following reaction.
for the electrochemical cell constructed using the following reaction. 
A) (+0.647 V)
B) (-0.647 V)
C) (+0.895 V)
D) (-0.895 V)
E) (-1.17 V)
Correct Answer:
Verified
Q66: The work involved in moving exactly 1
Q67: If the potential of a voltaic cell
Q68: Copper is oxidized by nitric acid. If
Q69: If the free-energy change of the following
Q70: The change in free energy for
Q72: The Nernst equation can be used to
Q73: If the free-energy change of a voltaic
Q74: Which statement does not correctly describe a
Q75: Does pH have an effect on the
Q76: The standard hydrogen electrode is _
A)used to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents