For the following reaction, predict the pH if the pressure of O2 is 3.00 atm and the bromide ion concentration is 2.10 M. The measured cell potential (Ecell) was 0.100 V. 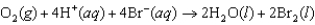
Half reactions: 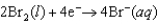 E = +1.077 V
E = +1.077 V
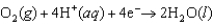 E = +1.229 V
E = +1.229 V
A) 4.31
B) 3.17
C) 2.59
D) 1.32
E) 0.66
Correct Answer:
Verified
Q86: An electrochemical cell at 298 K is
Q87: Lead is a toxic metal. The
Q88: Which statement about this concentration cell is
Q89: An electrochemical cell has both a silver
Q90: Electrochemical cell potentials can be used
Q92: When a voltaic cell reaches equilibrium, _
A)
Q93: What is the cell potential for this
Q94: A pH meter uses an electrode arrangement
Q95: Zinc-air batteries are actively being researched
Q96: Neuron cells generate electrical signals by concentration
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents