An electrochemical cell at 298 K is composed of two compartments, labeled C1 and C2. C1 consists of a copper metal electrode immersed in a 0.30 M copper sulfate solution.
C2 consists of a copper metal electrode immersed in a 1.5 M copper sulfate solution. 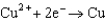 ,
, 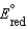 = + 0.34 V
= + 0.34 V
Which statement regarding this electrochemical cell is not correct?
A) The reaction continues until the copper ion concentrations in the two cells are equal.
B) The initial cell potential = 0.02 V.
C) The cell potential gradually decreases to 0.0 V.
D) The standard cell potential is +0.34 V.
E) Reduction occurs in compartment C2.
Correct Answer:
Verified
Q81: An electrochemical cell is represented below. Which
Q82: The standard cell potential for the nickel-cadmium
Q83: What is true when a battery (voltaic
Q84: Cadmium is a toxic heavy metal.
Q85: An electrochemical cell is constructed with
Q87: Lead is a toxic metal. The
Q88: Which statement about this concentration cell is
Q89: An electrochemical cell has both a silver
Q90: Electrochemical cell potentials can be used
Q91: For the following reaction, predict the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents