Determine the entropy change for the reaction 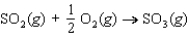 given the following information:
given the following information: 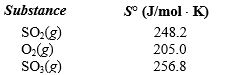
A) (-196.4 J/K)
B) (+196.4 J/K)
C) (-93.9 J/K)
D) (+93.9 J/K)
E) (+401.4 J/K)
Correct Answer:
Verified
Q49: The standard molar entropy of silver chloride
Q50: What is the entropy change if 4.500
Q51: Determine the standard entropy of N2(g)
Q52: Determine
Q53: What is the difference between
Q55: The symbol
Q56: Which of the following is in the
Q57: The standard molar entropy of lead(II) bromide
Q58: What is the standard entropy change when
Q59: Determine
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents