Determine the standard entropy of N2(g) given the following information: 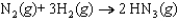
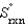 = -198.3 J/K
= -198.3 J/K 
A) (-260.2 J/K)
B) (+260.2 J/K)
C) (+93.9 J/K)
D) (+191.5 J/K)
E) (-191.5 J/K)
Correct Answer:
Verified
Q46: Indicate which of the following has
Q47: The symbol
Q48: Which of the relationships between the
Q49: The standard molar entropy of silver chloride
Q50: What is the entropy change if 4.500
Q52: Determine
Q53: What is the difference between
Q54: Determine the entropy change for the reaction
Q55: The symbol
Q56: Which of the following is in the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents