The reaction 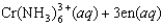
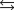
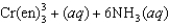 where en represents ethylenediamine, has a small value for the enthalpy change, Hrxn, yet the free-energy change is large because ________
where en represents ethylenediamine, has a small value for the enthalpy change, Hrxn, yet the free-energy change is large because ________
A) the reaction rate is fast.
B) the entropy change is large and positive.
C) the enthalpy change is large enough to matter.
D) the entropy change is large and negative.
E) ethylenediamine has amino groups that are stronger bases than ammonia.
Correct Answer:
Verified
Q70: Hydrochloric acid (HCl) reacts with sodium
Q71: Determine the value of
Q72: Determine the value of
Q73: Determine the value of
Q74: What is the maximum amount of work
Q76: A reaction is at equilibrium at
Q77: The symbol
Q78: Given the following data, determine the molar
Q79: The equilibrium vapor pressure for benzene
Q80: At constant T and P, any
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents