Which of the following objects will cool the fastest from the same initial temperature, assuming you had equal masses of each?
A) an aluminum pan (cs = 0.90 J/(g . 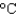 ) )
) )
B) a copper pot (cs = 0.39 J/(g . 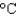 ) )
) )
C) an iron skillet (cs = 0.45 J/(g . 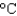 ) )
) )
D) a container of water (4.2 J/(g . 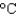 ) )
) )
E) a container of ethanol (2.5 J/(g .  ) )
) )
Correct Answer:
Verified
Q59: A heating curve for some substance is
Q60: Steam in a cylinder is compressed by
Q61: In an experiment, 30.0 g of
Q62: If 7.3 kJ of energy are required
Q63: Ammonium nitrate sometimes is used in cold
Q65: Given the following thermochemical equation detailing
Q66: When 1.14 g of octane (molar
Q67: A 15 g piece of iron
Q68: Given the following thermochemical equation detailing
Q69: When 2.50 g of sucrose (molar
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents