Given the following thermochemical equation detailing the combustion of methane CH4(g) 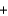 2O2(g)
2O2(g)  CO2(g)
CO2(g) 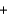 2H2O(g) Hrxn = -802kJ/mol CH4
2H2O(g) Hrxn = -802kJ/mol CH4
Determine the amount of energy released when 25.0 g of methane undergoes combustion.
A) 1.95 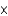
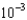 kJ
kJ
B) 3.12 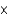
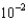 kJ
kJ
C) 453 kJ
D) 1250 kJ
E) 2.01 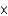
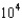 kJ
kJ
Correct Answer:
Verified
Q60: Steam in a cylinder is compressed by
Q61: In an experiment, 30.0 g of
Q62: If 7.3 kJ of energy are required
Q63: Ammonium nitrate sometimes is used in cold
Q64: Which of the following objects will cool
Q66: When 1.14 g of octane (molar
Q67: A 15 g piece of iron
Q68: Given the following thermochemical equation detailing
Q69: When 2.50 g of sucrose (molar
Q70: The cooling system in an automobile holds
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents