Given the following thermochemical equation detailing the combustion of glucose C6H12O6(s) 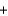 6O2(g)
6O2(g)  CO2(g)
CO2(g) 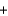 6H2O(g) Hrxn = -2803 kJ/mol C6-H12O6
6H2O(g) Hrxn = -2803 kJ/mol C6-H12O6
Determine the amount of glucose needed to release 30 kJ of energy.
A) 11.58 g
B) 1.93 g
C) 467 g
D) 1,930 g
E) 5.35 g
Correct Answer:
Verified
Q63: Ammonium nitrate sometimes is used in cold
Q64: Which of the following objects will cool
Q65: Given the following thermochemical equation detailing
Q66: When 1.14 g of octane (molar
Q67: A 15 g piece of iron
Q69: When 2.50 g of sucrose (molar
Q70: The cooling system in an automobile holds
Q71: In an experiment, 5.0 g of ice
Q72: A 150 g piece of iron (CP
Q73: In an experiment, 10.0 g of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents