In an experiment, 10.0 g of ice at -20 C is converted into steam with a temperature of 110 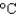 . How much energy is required for this process?
. How much energy is required for this process?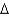
vap 2,260 J/g; 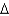 Hfus = 334 J/g; cs(ice) = 2.06 J/(g .
Hfus = 334 J/g; cs(ice) = 2.06 J/(g . 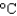 ) ; cs(water) = 4.18 J/(g .
) ; cs(water) = 4.18 J/(g . 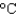 ) ; cs(steam) = 1.99 J/(g .
) ; cs(steam) = 1.99 J/(g .  ) )
) )
A) 30.7 kJ
B) 26.8 kJ
C) 34.9 kJ
D) 30.3 kJ
E) 38.7 kJ
Correct Answer:
Verified
Q68: Given the following thermochemical equation detailing
Q69: When 2.50 g of sucrose (molar
Q70: The cooling system in an automobile holds
Q71: In an experiment, 5.0 g of ice
Q72: A 150 g piece of iron (CP
Q74: The energy content of a Big Mac
Q75: You hold a 50 g sphere of
Q76: A food sample was burned in
Q77: You have a summer job in a
Q78: In an experiment, 74.3 g of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents