You have a summer job in a lead foundry. Your task is to identify how energy efficiency can be improved. You therefore need to know the minimum amount of energy it takes to raise 1 pound of lead (454 g) from room temperature (25 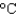 ) to its melting point (327
) to its melting point (327 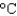 ) and then melt it. The specific heat capacity of lead is 0.159 J/(g .
) and then melt it. The specific heat capacity of lead is 0.159 J/(g .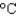 ) , the enthalpy of fusion is 24.7 J/g, and the molar mass is 207 g/mol.
) , the enthalpy of fusion is 24.7 J/g, and the molar mass is 207 g/mol.
A) 3.39 MJ
B) 21.9 kJ
C) 21.0 kJ
D) 33.0 kJ
E) 11.0 kJ
Correct Answer:
Verified
Q72: A 150 g piece of iron (CP
Q73: In an experiment, 10.0 g of
Q74: The energy content of a Big Mac
Q75: You hold a 50 g sphere of
Q76: A food sample was burned in
Q78: In an experiment, 74.3 g of
Q79: Using the following data for water, determine
Q80: In an experiment, 7.5 g of
Q81: Isooctane is a good model compound
Q82: Use the following information to determine
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents