In an experiment, 7.5 g of liquid water at 35 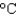 is converted into steam with a temperature of 145
is converted into steam with a temperature of 145
C. How much energy is required for this process?( 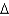 Hvap= 2,260 J/g;
Hvap= 2,260 J/g; 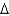 Hfus = 334 J/g; cs(ice) = 2.06 J/(g .
Hfus = 334 J/g; cs(ice) = 2.06 J/(g . 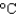 ) ; cs(water) = 4.18 J/(g .
) ; cs(water) = 4.18 J/(g .  ) ; cs(steam) = 1.99 J/(g .
) ; cs(steam) = 1.99 J/(g .  ) )
) )
A) 2.04 kJ
B) 17.0 kJ
C) 19.7 kJ
D) 5.40 kJ
E) 125 kJ
Correct Answer:
Verified
Q75: You hold a 50 g sphere of
Q76: A food sample was burned in
Q77: You have a summer job in a
Q78: In an experiment, 74.3 g of
Q79: Using the following data for water, determine
Q81: Isooctane is a good model compound
Q82: Use the following information to determine
Q83: The integrated circuits in your cell
Q84: Isooctane is a good model compound for
Q85: Which statement regarding combustion of a sample
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents