The cooling system in an automobile holds 10.0 L of ethylene glycol antifreeze. How much energy is absorbed when the temperature of the ethylene glycol goes from 20 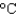 to 100
to 100 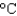 ? The density and specific heat capacity of ethylene glycol are 1.11 g/mL and 2.42 J/(g .
? The density and specific heat capacity of ethylene glycol are 1.11 g/mL and 2.42 J/(g .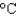 ) , respectively.
) , respectively.
A) 2,150 J
B) 2,150 kJ
C) 1,940 kJ
D) 1,940 J
E) 215 J
Correct Answer:
Verified
Q65: Given the following thermochemical equation detailing
Q66: When 1.14 g of octane (molar
Q67: A 15 g piece of iron
Q68: Given the following thermochemical equation detailing
Q69: When 2.50 g of sucrose (molar
Q71: In an experiment, 5.0 g of ice
Q72: A 150 g piece of iron (CP
Q73: In an experiment, 10.0 g of
Q74: The energy content of a Big Mac
Q75: You hold a 50 g sphere of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents