How many nitrogen atoms are there in 51.0 g of (NH4) 2SO4 (ammonium sulfate) ?
A) 2.32  1023 atoms
1023 atoms
B) 3.07 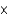 1025 atoms
1025 atoms
C) 4.65 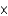 1023 atoms
1023 atoms
D) 6.14 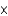 1025 atoms
1025 atoms
E) 5.39 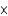 1023 atoms
1023 atoms
Correct Answer:
Verified
Q24: Fuming sulfuric acid is obtained by the
Q25: Air bags in cars inflate when an
Q26: Sulfur dioxide from coal-fired power plants combines
Q27: Fe2O3(s) and powdered aluminum can react with
Q28: Nitrogen monoxide undergoes combustion to produce nitrogen
Q30: What is the molar mass of sulfuric
Q31: Which of the following cartoons depict a
Q32: The acid-base reaction between phosphoric acid, H3PO4,
Q33: Which statement about the following chemical reaction
Q34: As a purchasing agent for a pharmaceutical
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents