Which statement about the following chemical reaction is not correct?
3H2 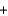
N2

2NH3
A) For every mole of nitrogen consumed, two moles of ammonia are produced.
B) For every two moles of nitrogen consumed, three moles of ammonia are produced.
C) For every three moles of hydrogen consumed, two moles of ammonia are produced.
D) For every two moles of ammonia produced, three moles of hydrogen are consumed.
E) Three moles of hydrogen will react with one mole of nitrogen to produce ammonia.
Correct Answer:
Verified
Q28: Nitrogen monoxide undergoes combustion to produce nitrogen
Q29: How many nitrogen atoms are there in
Q30: What is the molar mass of sulfuric
Q31: Which of the following cartoons depict a
Q32: The acid-base reaction between phosphoric acid, H3PO4,
Q34: As a purchasing agent for a pharmaceutical
Q35: Which statement about a balanced chemical reaction
Q36: What is the molar mass of phosphorus
Q37: What is the formula mass of (NH4)3PO4
Q38: Which of the following cartoons depict a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents