Nitrogen monoxide undergoes combustion to produce nitrogen dioxide. Which relationship regarding the quantities of reactants and products associated with this reaction is not correct? 2NO 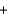 O2
O2  2NO2
2NO2
A) 2x g 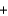 x g
x g  2x g
2x g
B) 2 molecules 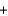 +1 molecule
+1 molecule  2 molecules
2 molecules
C) 60.0 g 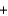 32.0 g
32.0 g  92.0 g
92.0 g
D) (2y) (30.0 g) 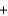 y (32.0 g)
y (32.0 g)  (2y) (46.0 g)
(2y) (46.0 g)
E) 15.0 g 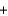 8.0 g
8.0 g  23.0 g
23.0 g
Correct Answer:
Verified
Q23: Hydrogen peroxide decomposes to produce water
Q24: Fuming sulfuric acid is obtained by the
Q25: Air bags in cars inflate when an
Q26: Sulfur dioxide from coal-fired power plants combines
Q27: Fe2O3(s) and powdered aluminum can react with
Q29: How many nitrogen atoms are there in
Q30: What is the molar mass of sulfuric
Q31: Which of the following cartoons depict a
Q32: The acid-base reaction between phosphoric acid, H3PO4,
Q33: Which statement about the following chemical reaction
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents