Indicate which one of the following reactions result in a positive Ssys.
A) AgNO3(aq) + NaCl(aq) AgCl(s) + NaNO3(aq)
B) HCl(g) + H2O( 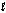 ) HCl(aq)
) HCl(aq)
C) H2(g) + I2(g) 2 Hl(g)
D) C2H2O2(g) 2 CO(g) + H2(g)
E) H2O(g) H2O(l)
Correct Answer:
Verified
Q3: What is the entropy change to the
Q7: If 1 mol of ice melts at
Q29: Which of the following is in the
Q30: The standard molar entropy of lead(II) bromide
Q31: The following figures represent distributions of two
Q33: Indicate which of the following has the
Q34: The following figures represent distributions of gas
Q37: Which of the following graphs best depicts
Q39: Indicate which one of the following
Q44: The entropy of a NaCl crystal is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents