Consider the following hypothetical equilibrium reaction: A2(g) + B2(g) ⇌ 2AB(g) where Kc = 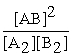 The equilibrium constant for the reaction: 2 A2(g) + 2 B2(g) ⇔ 4 AB(g) is ________.
The equilibrium constant for the reaction: 2 A2(g) + 2 B2(g) ⇔ 4 AB(g) is ________.
A) 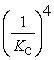
B) Kc4
C) 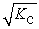
D) 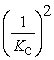
E) Kc2
Correct Answer:
Verified
Q73: For the reaction: 2 SO2(g) + O2(g)
Q74: Two moles of NH3 are initially present
Q75: Given the following reactions, 2PCl3(g) ⇌ 2P(g)
Q76: For the reaction: 3 Fe(s) + 4
Q77: Write the equilibrium constant expression for the
Q79: For the reaction: 3 Fe(s) + 4
Q80: In the reaction: 2 N2O(g) + N2H4(g)
Q81: 4.000 mol chlorine and 2.000 mol bromine
Q82: A mixture, containing 0.0750 M HCl(g) and
Q83: Consider the following reaction: POCl3(g) ⇌ POCl(g)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents