Write the equilibrium constant expression for the reaction: 3 Sn(s) + 4 HNO3(aq) + H2O(l) ⇌ 3 H2SnO3(s) + 4 NO(g)
A) Kc = 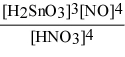
B) Kc = 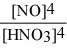
C) Kc = 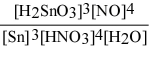
D) Kc = 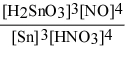
E) Kc = 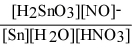
Correct Answer:
Verified
Q72: For the reaction: 3 Fe(s) + 4
Q73: For the reaction: 2 SO2(g) + O2(g)
Q74: Two moles of NH3 are initially present
Q75: Given the following reactions, 2PCl3(g) ⇌ 2P(g)
Q76: For the reaction: 3 Fe(s) + 4
Q78: Consider the following hypothetical equilibrium reaction: A2(g)
Q79: For the reaction: 3 Fe(s) + 4
Q80: In the reaction: 2 N2O(g) + N2H4(g)
Q81: 4.000 mol chlorine and 2.000 mol bromine
Q82: A mixture, containing 0.0750 M HCl(g) and
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents