Shown below is a phase diagram for compound X. At 25°C and 1 atm, in what state will X exist? 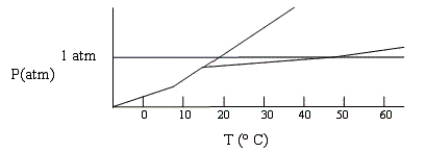
A) gas/solid at equilibrium.
B) liquid.
C) gas/liquid at equilibrium.
D) solid.
E) gas.
Correct Answer:
Verified
Q46: A certain metal fluoride crystallizes in such
Q63: \(\Delta\Hvap for water is 40.7 kJ/mol. Calculate
Q64: When 1.00 mol of a pure
Q64: What is the vapor pressure of water
Q68: A liquid placed in a closed container
Q68: A sample consisting of CO2(g) and CO2(s)
Q70: Below is a phase diagram for compound
Q71: The triple point of a substance is
A)
Q73: What is the vapor pressure of water
Q77: In which of the following processes is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents