Below is a phase diagram for compound X. What is the normal boiling point of X is most likely to be? 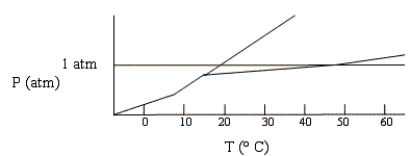
A) 0° C
B) 73° C
C) 15° C
D) 47° C
E) 18° C
Correct Answer:
Verified
Q65: Shown below is a phase diagram for
Q67: Which of the following statements is true
Q68: A sample consisting of CO2(g) and CO2(s)
Q68: A liquid placed in a closed container
Q71: The triple point of a substance is
A)
Q71: Given below are the temperatures at which
Q72: When 1.00 mol of a pure
Q73: What is the vapor pressure of water
Q74: The heat of vaporization of a
Q75: You are given the following boiling-point data:
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents