A sample consisting of CO2(g) and CO2(s) at equilibrium at -78°C and 1 atm pressure is heated to -30°C, and the pressure is increased to 8 atm. Based on the phase diagram below, what will happen? 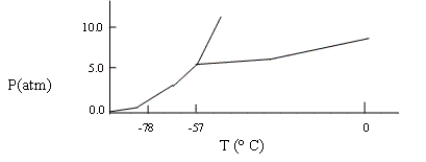
A) At equilibrium, only CO2(g) will be present.
B) The melting point of the CO2(s) will decrease.
C) At equilibrium, CO2(g) and CO2(l) will be present.
D) All the CO2 will be converted to CO2(l) .
E) none of these
Correct Answer:
Verified
Q63: \(\Delta\Hvap for water is 40.7 kJ/mol. Calculate
Q64: When 1.00 mol of a pure
Q65: Shown below is a phase diagram for
Q67: Which of the following statements is true
Q68: A liquid placed in a closed container
Q70: Below is a phase diagram for compound
Q71: The triple point of a substance is
A)
Q71: Given below are the temperatures at which
Q72: When 1.00 mol of a pure
Q73: What is the vapor pressure of water
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents