Calculate E at 25°C for this cell, given the following data: 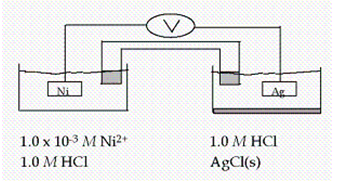 Ag+ + e- Ag(s) E° = 0.80 V
Ag+ + e- Ag(s) E° = 0.80 V
Ni2+ + 2e- Ni(s) E° = -0.23 V
Ksp for AgCl = 1.6 *10-10
A) 2.98 V
B) This cannot be determined from the data given
C) 0.83 V
D) 1.01 V
E) 0.54 V
Correct Answer:
Verified
Q71: An electrolytic cell process involves plating Zr(s)
Q73: An antique automobile bumper is to be
Q73: Why is aluminum protected from corrosion? (Note:
Q74: An antique automobile bumper is to be
Q75: Use the following data to calculate the
Q77: Copper is electroplated from an aqueous CuSO4
Q79: If an electrolysis plant operates its electrolytic
Q81: In the following equation
Q82: Which of the following would be a
Q83: The SI unit for current is the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents