Use the following data to calculate the Ksp value at 25°C for PbSO4(s) . 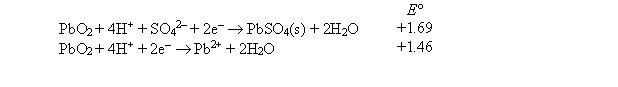
A) 3.89 * 10-105
B) 2.57 *10105
C) 1.7 * 10-8
D) 5.9* 107
E) None of these is within 5% of the correct answer.
Correct Answer:
Verified
Q70: Fe2+ + 2e-
Q71: What quantity of charge is required to
Q71: An electrolytic cell process involves plating Zr(s)
Q72: Electrolysis of a molten salt with the
Q73: An antique automobile bumper is to be
Q73: Why is aluminum protected from corrosion? (Note:
Q74: An antique automobile bumper is to be
Q77: Copper is electroplated from an aqueous CuSO4
Q78: Calculate E at 25°C for this
Q79: If an electrolysis plant operates its electrolytic
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents