A 0.50 M solution of hydrofluoric acid,HF,is 3.6% ionized.The acid ionization constant, Ka,for hydrofluoric acid is
A) 3.6 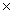 10-2
10-2
B) 1.8 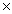 10-2
10-2
C) 3.2 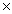 10-4
10-4
D) 6.5 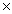 10-4
10-4
Correct Answer:
Verified
Q60: Hydrosulfuric acid,H2S,is a weak acid whose Ka
Q61: The equilibrium constant can change significantly with
Q62: Calculate the pH of a 0.250 M
Q63: The magnitude of Keq depends on
A)initial concentration
Q64: Calculate the percent ionization of a 0.200
Q66: Calculate the percent ionization of a 0.050
Q67: Which solution has the highest pH?
A)0.1 M
Q68: Which solution has the lowest pH?
A)0.1 M
Q69: What will be the [H+1] in a
Q70: The mathematical expression for the equilibrium constant
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents