Calculate the pH of a 0.250 M aqueous solution of HNO2.The acid dissociation constant,Ka,for HNO2 is 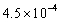 .
.
A) 1.97
B) 2.74
C) 3.35
D) 3.95
Correct Answer:
Verified
Q57: HCl is added to a saturated AgCl
Q58: What is the pH of a 0.0500
Q59: Which salt would produce a basic solution?
A)KBr
B)NaNO2
C)CuCl
D)NH4Cl
Q60: Hydrosulfuric acid,H2S,is a weak acid whose Ka
Q61: The equilibrium constant can change significantly with
Q63: The magnitude of Keq depends on
A)initial concentration
Q64: Calculate the percent ionization of a 0.200
Q65: A 0.50 M solution of hydrofluoric acid,HF,is
Q66: Calculate the percent ionization of a 0.050
Q67: Which solution has the highest pH?
A)0.1 M
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents