Consider the reaction: CH3CH2∙ + Br2 → CH3CH2Br + Br∙ .
Given that this reaction has an activation energy of +6 kcal/mol and a 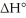
of 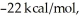
sketch a reaction-energy diagram for this reaction. Label the axes and show Ea and 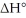
on your drawing.
Correct Answer:
Verified
Q72: _ is the minimum kinetic energy reacting
Q73: In the hydrocarbon shown below, how many
Q74: Consider the conversion of X to Z
Q75: Given an activation energy of 15 kcal/mol,
Q76: What term describes the highest-energy structure in
Q78: Consider the three-step mechanism for the reaction
Q79: The following reaction occurs readily: 
Q80: Provide the structure of the transition state
Q81: Rank the free radicals (I-III) shown below
Q82: When compound I, C7H16, was treated with
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents