A reaction has ΔG = 10.0 kJ and ΔG° = 15.0 kJ at a temperature of 50°C. Calculate the value of the reaction quotient 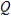 under these conditions.
under these conditions.
A) 0.16
B) 9.1 × 10 −5
C) 1.1 × 10 4
D) 6.4
E) 6.0 × 10 −6
Correct Answer:
Verified
Q75: Calculate ΔG° for the combustion of propane.
Q76: Nitric oxide reacts with chlorine to form
Q77: Consider the figure that shows ΔG° for
Q78: Calculate ΔG° for the reaction SiCl4(g) +
Q79: Consider the figure that shows ΔG° for
Q80: A reaction is proceeding toward equilibrium. At
Q81: Select the correct statement of a law
Q82: Consider the reaction CuI(s) ⇄ Cu+(aq) +
Q83: The formation constant for the reaction Ag+(aq)
Q84: In 1774 Joseph Priestley prepared the element
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents