Multiple Choice
The s, p, d, f, symbols represent values of the quantum number:
A) ms
B) n
C) 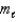
D) 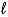
E) mj
Correct Answer:
Verified
Related Questions
Q5: Of the following states, 5s, 3p, 4f,
Q13: In a shell of the hydrogen atom
Q14: The energy needed to remove an electron
Q15: The K, L, M symbols represent values
Q16: The number of states in the He+
Q19: If P(r) is the radial probability density
Q20: The energy needed to change a He+
Q21: In 1921, Stern and Gerlach performed an
Q22: In terms of a0, where a0 =
Q40: The Pauli Exclusion Principle states
A) no two
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents