In a shell of the hydrogen atom with n = 3, the permitted values of the orbital magnetic quantum number are: 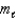
A) (-1, 0, 1)
B) 2, 1, 0
C) 2, 1, 0, -1, -2
D) 0
E) 3, 2, 1, 0, -1, -2, -3
Correct Answer:
Verified
Q5: Of the following states, 5s, 3p, 4f,
Q8: The probability density of a particle at
Q9: For the following allowed transitions, which photon
Q11: An energy of 13.6 eV is needed
Q12: The radial portion of the de
Q14: The energy needed to remove an electron
Q15: An electron in a hydrogen atom makes
Q15: The K, L, M symbols represent values
Q16: The number of states in the He+
Q18: The s, p, d, f, symbols represent
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents