Multiple Choice
The K, L, M symbols represent values of the quantum number:
A) n
B) 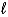
C) 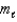
D) ms
E) mj
Correct Answer:
Verified
Related Questions
Q5: Of the following states, 5s, 3p, 4f,
Q11: An energy of 13.6 eV is needed
Q12: The radial portion of the de
Q13: In a shell of the hydrogen atom
Q14: The energy needed to remove an electron
Q15: An electron in a hydrogen atom makes
Q16: The number of states in the He+
Q18: The s, p, d, f, symbols represent
Q19: If P(r) is the radial probability density
Q20: The energy needed to change a He+
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents