When the reversible reaction, N₂+ O₂ 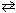 2 NO
2 NO
Has reached a state of dynamic equilibrium , which statement(s) below is(are) true?
I. Both the forward and reverse reactions shut down and no more NO, N₂or O₂are produced.
II. The rate of the forward reaction equals the rate of the reverse reaction.
III. The rate constant of the forward reaction equals the rate constant of the reverse reaction.
A) I only
B) II only
C) III only
D) I and II
E) All of these.
Correct Answer:
Verified
Q1: The equilibrium constant for the reaction below
Q2: The equilibrium constant, K c , for
Q3: Consider the following reaction and its corresponding
Q5: Consider the following reaction and its corresponding
Q6: Given the following two reactions and their
Q7: Given the following two reactions and their
Q8: Given the following information, HF (aq)
Q9: The equilibrium constant for the reaction below
Q10: At 400 C the value of K
Q11: Given:
2 NO (g) + 2 H₂ (g)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents