Consider the following reaction and its corresponding equilibrium constant:
2 ICl (g) 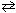 I2 (g) + Cl₂ (g) K c1 = 0.110 What would be the equilibrium constant for the following reaction?
I2 (g) + Cl₂ (g) K c1 = 0.110 What would be the equilibrium constant for the following reaction?
ICl (g) 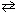 1 / 2 I2 (g) + 1 / 2 Cl₂ (g) K c2 = ??
1 / 2 I2 (g) + 1 / 2 Cl₂ (g) K c2 = ??
A) 0.0121
B) 0.0550
C) 0.110
D) 0.220
E) 0.332
Correct Answer:
Verified
Q1: The equilibrium constant for the reaction below
Q2: The equilibrium constant, K c , for
Q4: When the reversible reaction, N₂+ O₂
Q5: Consider the following reaction and its corresponding
Q6: Given the following two reactions and their
Q7: Given the following two reactions and their
Q8: Given the following information, HF (aq)
Q9: The equilibrium constant for the reaction below
Q10: At 400 C the value of K
Q11: Given:
2 NO (g) + 2 H₂ (g)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents