The equilibrium constant for the reaction N₂O4 (g) 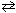 2 NO₂ (g) is 0.100 at 25 C. Calculate D G for this reaction. (R = 8.314 J/mol K)
2 NO₂ (g) is 0.100 at 25 C. Calculate D G for this reaction. (R = 8.314 J/mol K)
A) - 5.70 kJ/mol
B) +5.70 kJ/mol
C) - 0.479 kJ/mol
D) +0.479 kJ/mol
E) 0.00 kJ/mol
Correct Answer:
Verified
Q80: Exhibit 17-5 Consider the reaction of Barium
Q81: Given D G rxn = - 120.4
Q82: For any system at equilibrium, the value
Q83: Consider the following reaction:
2 HCl (g)
Q84: Calculate K p for the evaporation of
Q86: What is the value of the equilibrium
Q87: At 100 C, K p is 2.65
Q88: The equilibrium constant for the reaction PCl5
Q89: For 2 N2O (g) + O2 (g)→4
Q90: Given K p = 0.100 at 25
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents