Calculate K p for the evaporation of water at 25 C, given that D G = +8.6 kJ/mol for the process:
H₂ O ( 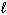 )
) 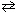 H₂ O (g)
H₂ O (g)
A) 32.2
B) 0.997
C) 4.1×10 - 2
D) 3.11×10 - 4
E) 3.1×10 - 2
Correct Answer:
Verified
Q79: Consider the conversion of ozone, O3, to
Q80: Exhibit 17-5 Consider the reaction of Barium
Q81: Given D G rxn = - 120.4
Q82: For any system at equilibrium, the value
Q83: Consider the following reaction:
2 HCl (g)
Q85: The equilibrium constant for the reaction N₂O4
Q86: What is the value of the equilibrium
Q87: At 100 C, K p is 2.65
Q88: The equilibrium constant for the reaction PCl5
Q89: For 2 N2O (g) + O2 (g)→4
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents