Given D G rxn = - 120.4 kJ/mol for the following reaction, what is the value of its equilibrium constant, K , at 298 K?
H₂ (g) + O₂(g) 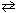 H₂ O₂ (
H₂ O₂ ( 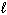 )
)
A) K = 7.7×10 - 22
B) K = 1.1
C) K = 49
D) K = 2.9×101
E) K = 1.3×1021
Correct Answer:
Verified
Q76: Exhibit 17-4 Consider the reaction of nitrogen
Q77: The calculated value of D S is
Q78: The reaction of ammonia and hydrogen chloride
Q79: Consider the conversion of ozone, O3, to
Q80: Exhibit 17-5 Consider the reaction of Barium
Q82: For any system at equilibrium, the value
Q83: Consider the following reaction:
2 HCl (g)
Q84: Calculate K p for the evaporation of
Q85: The equilibrium constant for the reaction N₂O4
Q86: What is the value of the equilibrium
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents