Multiple Choice
Use 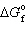 N₂= 0. kJ/mol,
N₂= 0. kJ/mol, 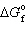 O₂= 0. kJ/mol,
O₂= 0. kJ/mol, 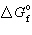 NO = 87 kJ/mol, and R = 8.314 J/mol K to calculate the standard free energy change at 298 K for:
NO = 87 kJ/mol, and R = 8.314 J/mol K to calculate the standard free energy change at 298 K for:
N₂(g, 5 atm) + O₂(g, 2 atm) 2 NO (g, 0.10 atm)
A) 70 kJ
B) 76 kJ
C) 157 kJ
D) 163 kJ
E) 191 kJ
Correct Answer:
Verified
Related Questions
Q96: Given that the dissociation constant, K a,
Q97: The equilibrium constant, K p , for
Q98: For a certain chemical reaction at 25
Q99: At 25 ° C, K p =
Q100: For the reaction N₂(g) + 3 H₂
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents