Passage
ATP synthase, also known as Complex V, produces the majority of the ATP in eukaryotic cells. It is a transmembrane protein encoded by both nuclear and mitochondrial genes in humans, and is found in the inner mitochondrial membrane. Complex V consists of over 20 subunits, including three sets of heterodimers arranged in a circle in the mitochondrial matrix. Each dimer contains an α subunit and a β subunit, and each β subunit exists in one of three conformations: one that binds ATP, one that binds ADP and inorganic phosphate, and one that binds neither.As protons flow from the intermembrane space through Complex V and into the matrix, they cause the transmembrane γ subunit to rotate and interact with each β subunit in turn. Four protons provide enough energy for rotation from one β subunit to the next, and each rotation causes a β subunit to release a new ATP molecule as it changes from the ATP-binding to the nonbinding conformation (Figure 1) . Thus 12 protons produce 3 ATP molecules each time the γ subunit makes a complete revolution. Every NADH molecule that enters the electron transport chain (ETC) pumps 10 protons into the intermembrane space for use by Complex V whereas only 6 protons are pumped per FADH2 molecule.
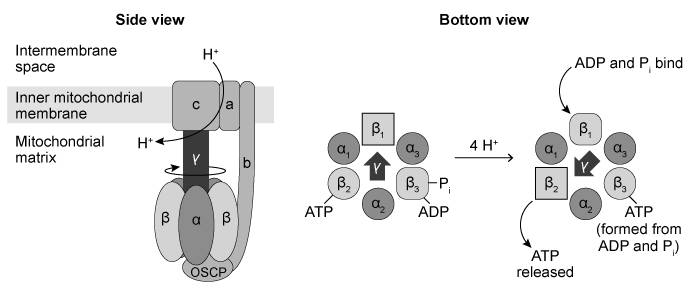 Figure 1 Depictions of ATP synthase viewed from the side (left) and the bottom (right) Researchers visualized the rotation of the γ subunit by attaching it to a fluorescently labeled actin filament. The observed rotation over time is shown in Figure 2.
Figure 1 Depictions of ATP synthase viewed from the side (left) and the bottom (right) Researchers visualized the rotation of the γ subunit by attaching it to a fluorescently labeled actin filament. The observed rotation over time is shown in Figure 2.
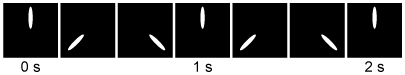 Figure 2 Counterclockwise rotation of the γ subunit of ATP synthase (bottom view) over time in seconds as visualized by a fluorescent labelAn L156P mutation in subunit A of Complex V is linked to neuropathy, ataxia, and retinal degeneration. Although the exact molecular bases of these pathological states are unknown, the following hypotheses are currently under study:Hypothesis 1L156P Complex V functions normally but is less abundant than wild-type Complex V.Hypothesis 2The mutation partially decouples proton flow from ATP synthesis.Hypothesis 3The mutation leads to increased oxidative stress from the ETC, resulting in apoptosis.
Figure 2 Counterclockwise rotation of the γ subunit of ATP synthase (bottom view) over time in seconds as visualized by a fluorescent labelAn L156P mutation in subunit A of Complex V is linked to neuropathy, ataxia, and retinal degeneration. Although the exact molecular bases of these pathological states are unknown, the following hypotheses are currently under study:Hypothesis 1L156P Complex V functions normally but is less abundant than wild-type Complex V.Hypothesis 2The mutation partially decouples proton flow from ATP synthesis.Hypothesis 3The mutation leads to increased oxidative stress from the ETC, resulting in apoptosis.
Adapted from Kucharczyk R, Zick M, Bietenhader M, et al. Mitochondrial ATP synthase disorders: molecular mechanisms and the quest for curative therapeutic approaches. Biochim Biophys Acta. 2009;1793(1) :186-99.
-All of the following could confirm apoptosis due to increased oxidative stress in cells with an L156P mutation in Complex V EXCEPT:
A) inhibition of caspase activity.
B) increased cytosolic cytochrome C.
C) activation of proteolysis.
D) increased mitochondrial superoxide concentration.
Correct Answer:
Verified
Q101: Passage
ATP synthase, also known as Complex V,
Q102: Passage
A newborn girl is diagnosed with a
Q103: Passage
Human gamma-glutamyl transpeptidase (hGGT) is an enzyme
Q104: Passage
ATP synthase, also known as Complex V,
Q105: Passage
Human gamma-glutamyl transpeptidase (hGGT) is an enzyme
Q107: Passage
Human gamma-glutamyl transpeptidase (hGGT) is an enzyme
Q108: Atpenins are succinate-ubiquinone reductase inhibitors with antifungal
Q109: Passage
Human gamma-glutamyl transpeptidase (hGGT) is an enzyme
Q110: Which of the following regulatory mechanisms helps
Q111: Researchers isolate a protein that runs as
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents