Passage
ATP synthase, also known as Complex V, produces the majority of the ATP in eukaryotic cells. It is a transmembrane protein encoded by both nuclear and mitochondrial genes in humans, and is found in the inner mitochondrial membrane. Complex V consists of over 20 subunits, including three sets of heterodimers arranged in a circle in the mitochondrial matrix. Each dimer contains an α subunit and a β subunit, and each β subunit exists in one of three conformations: one that binds ATP, one that binds ADP and inorganic phosphate, and one that binds neither.As protons flow from the intermembrane space through Complex V and into the matrix, they cause the transmembrane γ subunit to rotate and interact with each β subunit in turn. Four protons provide enough energy for rotation from one β subunit to the next, and each rotation causes a β subunit to release a new ATP molecule as it changes from the ATP-binding to the nonbinding conformation (Figure 1) . Thus 12 protons produce 3 ATP molecules each time the γ subunit makes a complete revolution. Every NADH molecule that enters the electron transport chain (ETC) pumps 10 protons into the intermembrane space for use by Complex V whereas only 6 protons are pumped per FADH2 molecule.
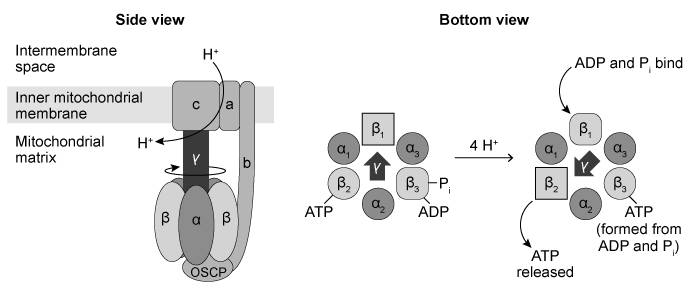 Figure 1 Depictions of ATP synthase viewed from the side (left) and the bottom (right) Researchers visualized the rotation of the γ subunit by attaching it to a fluorescently labeled actin filament. The observed rotation over time is shown in Figure 2.
Figure 1 Depictions of ATP synthase viewed from the side (left) and the bottom (right) Researchers visualized the rotation of the γ subunit by attaching it to a fluorescently labeled actin filament. The observed rotation over time is shown in Figure 2.
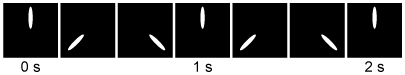 Figure 2 Counterclockwise rotation of the γ subunit of ATP synthase (bottom view) over time in seconds as visualized by a fluorescent labelAn L156P mutation in subunit A of Complex V is linked to neuropathy, ataxia, and retinal degeneration. Although the exact molecular bases of these pathological states are unknown, the following hypotheses are currently under study:Hypothesis 1L156P Complex V functions normally but is less abundant than wild-type Complex V.Hypothesis 2The mutation partially decouples proton flow from ATP synthesis.Hypothesis 3The mutation leads to increased oxidative stress from the ETC, resulting in apoptosis.
Figure 2 Counterclockwise rotation of the γ subunit of ATP synthase (bottom view) over time in seconds as visualized by a fluorescent labelAn L156P mutation in subunit A of Complex V is linked to neuropathy, ataxia, and retinal degeneration. Although the exact molecular bases of these pathological states are unknown, the following hypotheses are currently under study:Hypothesis 1L156P Complex V functions normally but is less abundant than wild-type Complex V.Hypothesis 2The mutation partially decouples proton flow from ATP synthesis.Hypothesis 3The mutation leads to increased oxidative stress from the ETC, resulting in apoptosis.
Adapted from Kucharczyk R, Zick M, Bietenhader M, et al. Mitochondrial ATP synthase disorders: molecular mechanisms and the quest for curative therapeutic approaches. Biochim Biophys Acta. 2009;1793(1) :186-99.
-If the pathological effects observed in patients with the L156P mutation are caused by decreased Complex V activity, the effects could be best counteracted by a drug that increases which of the following?
A) Malate-aspartate shuttle activity to transfer cytosolic NADH to the mitochondria
B) Potassium concentration in the mitochondrial matrix to decrease the charge gradient
C) The pH of the intermembrane space to equalize it with the pH of the matrix
D) Electron transfer from NADH to FAD via reduction of glycerol 3-phosphate to DHAP
Correct Answer:
Verified
Q96: Studies on the catalytic activity of hexokinase
Q97: Acetylation of lysine residues in histones increases
Q98: Passage
Synthesis of coenzyme A (CoA) requires vitamin
Q99: Passage
Myalgic encephalopathy (ME), or chronic fatigue syndrome
Q100: Passage
Myalgic encephalopathy (ME), or chronic fatigue syndrome
Q102: Passage
A newborn girl is diagnosed with a
Q103: Passage
Human gamma-glutamyl transpeptidase (hGGT) is an enzyme
Q104: Passage
ATP synthase, also known as Complex V,
Q105: Passage
Human gamma-glutamyl transpeptidase (hGGT) is an enzyme
Q106: Passage
ATP synthase, also known as Complex V,
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents