Consider the neutralization curve of aqueous ammonia with 0.1 M HCl, shown below. 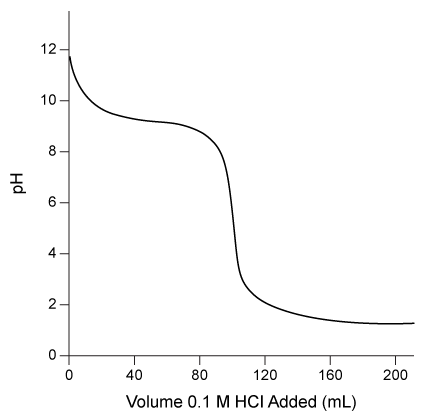 The best indicator to use in the titration is:
The best indicator to use in the titration is:
A) thymol blue (changes color from pH 1.2 to 2.8) .
B) methyl red (changes color from pH 4.4 to 6.2) .
C) phenolphthalein (changes color from pH 8.2 to 10.0) .
D) nitramine (changes color from pH 10.8 to 13.0) .
Correct Answer:
Verified
Q193: Passage
Students were asked to bring items from
Q194: The primary chemical composition of kidney stones
Q195: Which of the following molecules has the
Q196: Passage
Students were asked to bring items from
Q197: Passage
Students were asked to bring items from
Q199: Fructose 6-phosphate (Fru-6-P) breaks down in water
Q200: Passage
Students were asked to bring items from
Q201: Passage
Hyperbaric oxygenation therapy involves placing a patient
Q202: Which of the following statements describe a
Q203: Passage
Copper plays a vital role as a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents