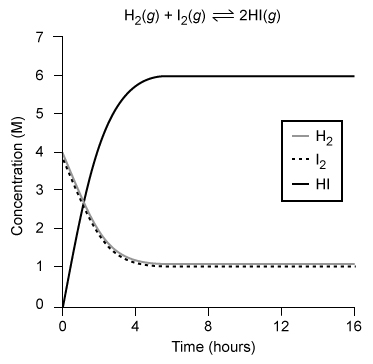
 Equilibrium is reached in a reversible reaction when the rate of the forward reaction equals the rate of the reverse reaction. The graph shows the changes in concentration during the formation of HI(g) from a mixture of H2(g) and I2(g) . If the measurement is taken at equilibrium, how many moles of HI(g) are present in a 750 mL sample of the reaction mixture?
Equilibrium is reached in a reversible reaction when the rate of the forward reaction equals the rate of the reverse reaction. The graph shows the changes in concentration during the formation of HI(g) from a mixture of H2(g) and I2(g) . If the measurement is taken at equilibrium, how many moles of HI(g) are present in a 750 mL sample of the reaction mixture?
A) 0.8 mol
B) 1.9 mol
C) 4.5 mol
D) 6.0 mol
Correct Answer:
Verified
Q233: The conjugate acid of the dihydrogen orthosilicate
Q234: Passage
Within the renal system, two buffers operate
Q235: The emission line spectrum of hydrogen displays
Q236: Passage
Amino acids and copper both serve vital
Q237: Passage
Living organisms that require oxygen to respire
Q239: Passage
Living organisms that require oxygen to respire
Q240: Passage
Amino acids and copper both serve vital
Q241: Passage
The tendency of a chemical species to
Q242: Passage
The radiopharmaceutical 2-deoxy-2-(18F)fluoro-D-glucose (abbreviated as 18F-FDG) is
Q243: Passage
The tendency of a chemical species to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents