Passage
The tendency of a chemical species to undergo reduction during an electrochemical reaction is indicated by the standard potential E° for the reaction. As represented generally by Reaction 1, E° measures the potential Z at which element X with oxidation number N accepts N electrons and is reduced to its elemental state.X(N) + Ne− → X(0) , E° = Z voltsReaction 1In a galvanic cell, E° > 0 indicates a spontaneous reaction. Therefore, chemical species with higher positive E° values are reduced more easily than those with lower E° values.Using E° as a basis of comparison, the relative thermodynamic stabilities of different chemical species involving the same element at different oxidation states can be presented graphically using a Frost diagram. For a series of compounds containing element X, a Frost diagram plots the value of NE° for each compound against the corresponding oxidation number N of element X within the species. Frost diagrams for several species containing manganese or chlorine are shown in Figure 1.
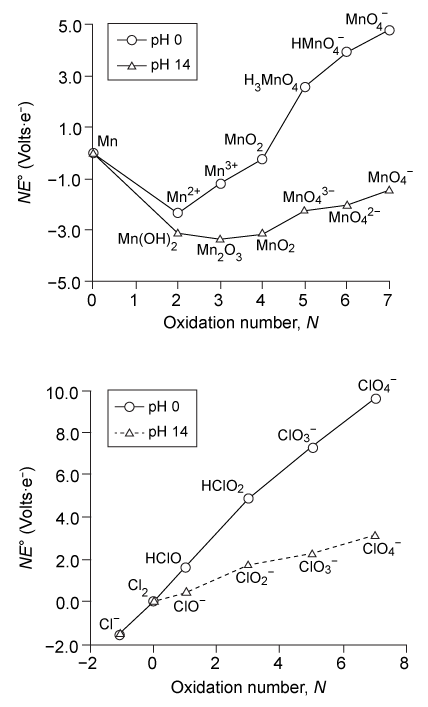 Figure 1 Frost diagrams for manganese and chlorineOn a Frost diagram, NE° is proportional to the standard Gibbs free energy ΔG° according to the relationshipΔG° = −FNE° = −nFE°Equation 1where n is the number of moles of electrons transferred during the electrochemical process and F is the Faraday constant. Free energy considerations also show that a species is prone to disproportionation if its position on the Frost diagram lies above a line connecting the points of two adjacent species, as seen for species B in Figure 2.
Figure 1 Frost diagrams for manganese and chlorineOn a Frost diagram, NE° is proportional to the standard Gibbs free energy ΔG° according to the relationshipΔG° = −FNE° = −nFE°Equation 1where n is the number of moles of electrons transferred during the electrochemical process and F is the Faraday constant. Free energy considerations also show that a species is prone to disproportionation if its position on the Frost diagram lies above a line connecting the points of two adjacent species, as seen for species B in Figure 2.
 Figure 2 General Frost diagram for an element forming species A, B, C, and D.The slope of a line segment joining two species on a Frost diagram is equal to the standard reduction potential for the couple. A greater slope indicates a higher corresponding reduction potential. As a result, in Figure 2, the reduction potential for B to A is lower than that for D to C.
Figure 2 General Frost diagram for an element forming species A, B, C, and D.The slope of a line segment joining two species on a Frost diagram is equal to the standard reduction potential for the couple. A greater slope indicates a higher corresponding reduction potential. As a result, in Figure 2, the reduction potential for B to A is lower than that for D to C.
-Based on the passage, which of the following species is LEAST likely to undergo a disproportionation reaction?
A) H3MnO4, pH = 0
B) MnO43−, pH = 14
C) Mn3+, pH = 0
D) MnO2, pH = 14
Correct Answer:
Verified
Q236: Passage
Amino acids and copper both serve vital
Q237: Passage
Living organisms that require oxygen to respire
Q238: Q239: Passage Q240: Passage Q242: Passage Q243: Passage Q244: A scientist uses extreme temperature environments to Q245: Passage Q246: Passage![]()
Living organisms that require oxygen to respire
Amino acids and copper both serve vital
The radiopharmaceutical 2-deoxy-2-(18F)fluoro-D-glucose (abbreviated as 18F-FDG) is
The tendency of a chemical species to
The tendency of a chemical species to
The radiopharmaceutical 2-deoxy-2-(18F)fluoro-D-glucose (abbreviated as 18F-FDG) is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents