A large, multinational drug corporation conducts a phase 1 clinical trail to evaluate the safety profile and pharmacokinetics properties of a new drug designed to treat refractory epilepsy. Initial studies showed that the drug undergoes extensive metabolism by the liver into glucuronidation byproducts that are primarily excreted by the kidneys. The curve bellow demonstrates the glucuronidation rate of the drug over a wide range of doses. 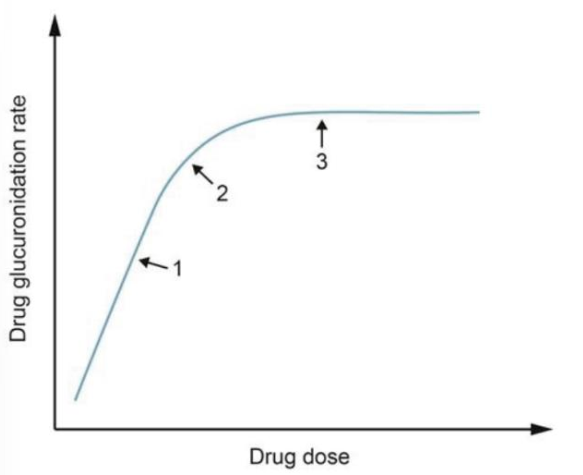
A) A constant proportion of the drug is metabolized past point 3
B) Bioavailability of the drug is highest at point 1
C) Biotransformation of the drug ceases near point 2
D) Metabolism begins to switch to zero-order kinetics near point 2
E) The rate of drug metabolism is not dependent on dose before point 1
Correct Answer:
Verified
Q77: A 49-year-old man with diabetes mellitus takes
Q78: An 80-year-old male nursing home resident is
Q79: A 44-year-old black male is brought to
Q80: Regarding the use of a daily baby
Q81: When comparing the administration of local anesthesia
Q82: Drug A and Drug B are of
Q83: A new vasopressor in development, Drug X,
Q84: Healthy adult volunteers are enrolled in a
Q86: Researchers are developing a new glycopeptide antibiotic
Q87: Researchers develop a novel glycopeptide antibiotic similar
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents