The reaction of a mixture of SO2 and O2 at a given temperature is represented by the equation
2SO2(g) + O2(g) 
2SO3(g)
When equilibrium is established,which of the following ratios is constant regardless of the initial concentrations of SO2 and O2?
A) 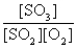
B) 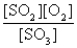
C) 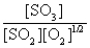
D) 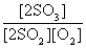
E) 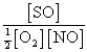
Correct Answer:
Verified
Q1: Sulfur dioxide combines with O2 in the
Q2: Nitrogen trifluoride decomposes to form nitrogen and
Q3: Which of the following represents a dynamic
Q4: Which of the following statements is true
Q6: The Ostwald process converts ammonia (NH3)to nitric
Q7: At 400 K,an equilibrium mixture of H2,I2,and
Q8: For the reaction Br2(g)+ Cl2(g) 
Q9: A 2.50-mol sample of HI is placed
Q10: A sample of ammonia gas was allowed
Q11: The following reaction is investigated (assume an
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents