Carbon tetrachloride reacts at high temperatures with oxygen to produce two toxic gases, phosgene and chlorine. CCl4(g) + 1/2O2(g) 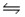
COCl2(g) + Cl2(g) , Kc = 4.4 × 109 at 1,000 K
Calculate Kc for the reaction 2CCl4(g) + O2(g) 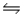
2COCl2(g) + 2Cl2(g) .
A) 4.4 × 109
B) 8.8 × 109
C) 1.9 × 1010
D) 1.9 × 1019
E) 2.3 × 10-10
Correct Answer:
Verified
Q14: When the following reaction is at equilibrium,
Q15: On analysis, an equilibrium mixture for the
Q16: The equilibrium between carbon dioxide gas and
Q17: When the following reaction is at equilibrium,
Q18: Given the following information: 2A(g)+ B(g)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents
