Multiple Choice
Which of these situations will result if some CH4(g) is removed from the reaction CO(g) + 3H2(g) 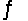 CH4(g) + H2O(g) at equilibrium?
CH4(g) + H2O(g) at equilibrium?
A) H2O will be consumed.
B) More CH4 and H2O will be produced.
C) Kp will decrease.
D) More CO will be produced.
E) No change will occur.
Correct Answer:
Verified
Related Questions
Q42: In which of these gas-phase equilibria is


